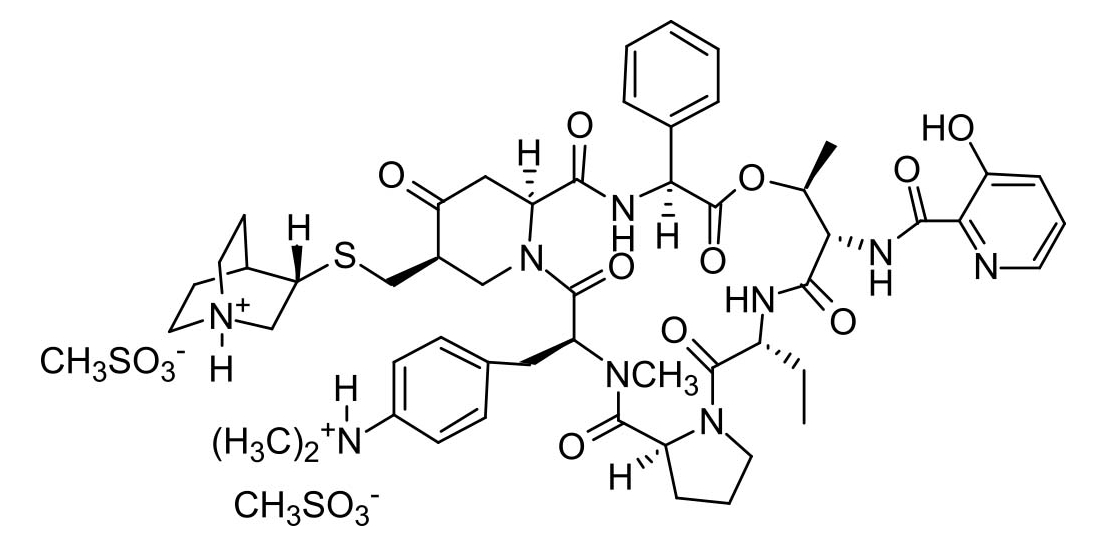
Quinupristin mesylate
Application Notes
Quinupristin is a semi-synthetic analogue of virginiamycin B (ostreogrycin B, pristinamycin IA, streptogramin B) formed by a Mannich condensation and elimination to generate an exocyclic methylene alpha to the ketone of the 4-piperidinone. Addition of quinucidinylthiol to the methylene group affords quinupristin. The structural changes provide a more hydrophobic compound with a readily ionisable group for generating a salt. Quinupristin is used commercially in synergistic combination with dalfopristin (30:70). There is little published data on the synthesis, biological or antibiotic activity of quinupristin alone, however the combination product is highly effective, including activity against antibiotic resistant strains.
References
- Quinupristin/dalfopristin (RP 59500): A new streptogramin antibiotic. Chant C. et al., Ann. Pharm. 1995, 29,1022.
- Quinupristin/dalfopristin: spectrum of activity, pharmacokinetics, and initial chemical experience. Low D. et al., Microb. Drug Resist, 1995, 1, 223.
- Quinupristin/dalfopristin: a therapeutic review. Allington D.R. & Rivey M.P. Clin. Ther. 2001, 23, 24.